Let’s go through some study results
TREMFYA® has been clinically studied and proven effective at helping patients with moderate to severe plaque psoriasis achieve clearer skin and fewer symptoms.
See photos below
90% CLEARER SKIN AT 16 WEEKS
In clinical studies, at 16 weeks, at least 7 out of 10 patients saw at least 90% clearer skin.
100% CLEARER SKIN AT 16 WEEKS
In one clinical study, 5 out of 10 patients saw completely clear skin at 16 weeks.
IMPROVED SYMPTOMS AT 16 WEEKS
In clinical studies, at 16 weeks, patients had improvements in symptoms of plaque psoriasis including itching, burning, pain, stinging, and skin tightness.
CLEAR OR ALMOST CLEAR SCALP AT 16 WEEKS
In clinical studies, at week 16, 4 out of 5 people with scalp psoriasis were rated clear or almost clear of their scalp psoriasis.
90% CLEARER SKIN ACROSS ALL SKIN TONES
In another study across all skin tones,* nearly 6 out of 10 patients saw 90% clearer skin at 16 weeks. Results may vary.
*Refers to a dermatology skin tone scale.
In a group of patients with moderate to severe scalp psoriasis, 6 out of 10 patients had at least 90% scalp clearance with nearly all of them 100% clear at 16 weeks. Results may vary.
90% CLEARER SKIN AT 48 WEEKS
In a clinical study, nearly 9 out of 10 people who saw 90% clearer skin at 28 weeks continued to see 90% clearer skin at 48 weeks.
90% CLEARER SKIN AT 5 YEARS
In a study, nearly 7 out of 10 patients with 90% clearer skin at 16 weeks were still clearer at 5 years.
At 1 year and thereafter, patients and healthcare providers knew that TREMFYA® was being used. This may have increased results. Results may vary.
CLEAR OR ALMOST CLEAR SKIN IN LOW-BSA MODERATE PLAQUE PSORIASIS AND HIGH-IMPACT AREAS*
In another study, at least 7 out of 10 patients were rated as clear or almost clear of their psoriasis at 16 weeks. Results may vary.
*High-impact areas have more sensitive skin and can be highly visible, like the face, scalp, skin folds, and genitals. A unique study with TREMFYA® showed that clearer skin is possible in patients with low body surface area (BSA) (2%-15%) moderate plaque psoriasis and plaques in these high-impact areas.
Individual results may vary.
Not everybody’s plaque psoriasis looks the same.
Watch the video to see how TREMFYA®
sees skin differently.
Before & After TREMFYA® Across All Skin Tones
See below for real patient photos before and after TREMFYA® treatment from the first study of its kind to include all skin tones.*
*Refers to a dermatology skin tone scale.
Photos are of real patients with moderate to severe plaque psoriasis who received a single dose of TREMFYA® 100 mg at weeks 0 and 4, and then every 8 weeks. Individual results may vary.
Identifies as African (Black)
Before
TREMFYA®

After 3 doses
WEEK 16
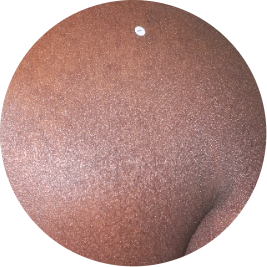
Back
Actual patient
Identifies as Caribbean (Black)
Before
TREMFYA®
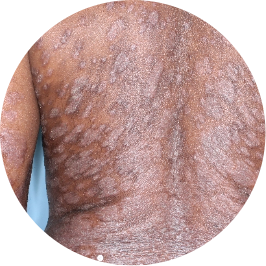
After 3 doses
WEEK 16
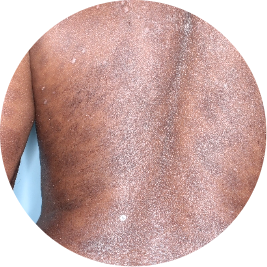
Back
Actual patient
Identifies as South American (Hispanic/Latino)
Before
TREMFYA®
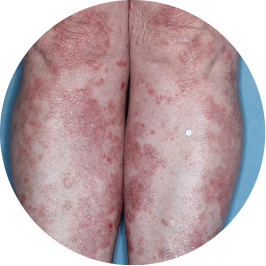
After 3 doses
WEEK 16
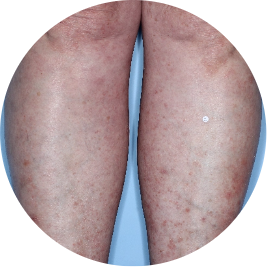
Legs
Actual patient
Identifies as Mexican (Hispanic/Latino)
Before
TREMFYA®
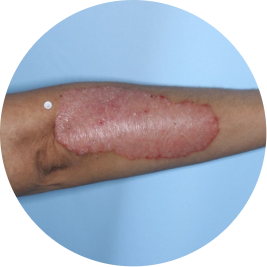
After 3 doses
WEEK 16
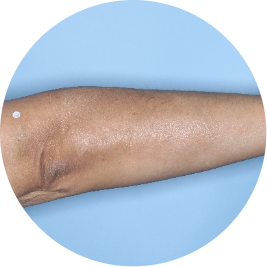
Arm
Actual patient
Identifies as Black
Before
TREMFYA®
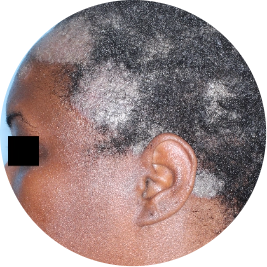
After 3 doses
WEEK 16
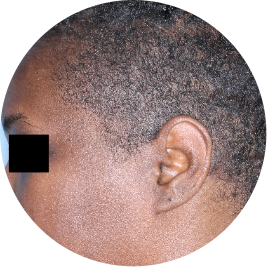
Scalp
Actual patient
Identifies as Caribbean (Black)
Before
TREMFYA®
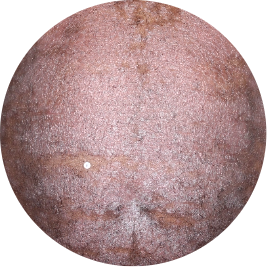
After 3 doses
WEEK 16
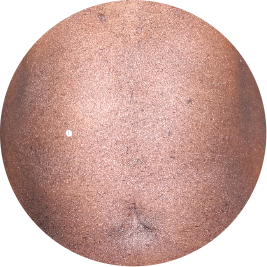
Chest
Actual patient
Identifies as Mexican (Hispanic/Latino)
Before
TREMFYA®
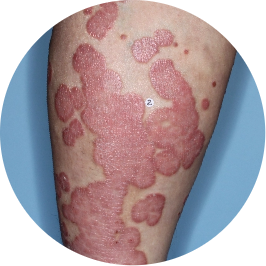
After 3 doses
WEEK 16
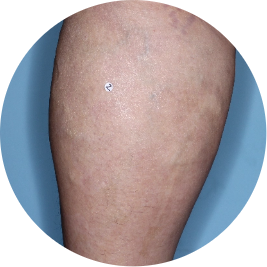
Leg
Actual patient
Identifies as Mexican (Hispanic/Latino)
Before
TREMFYA®
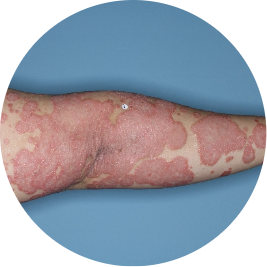
After 3 doses
WEEK 16
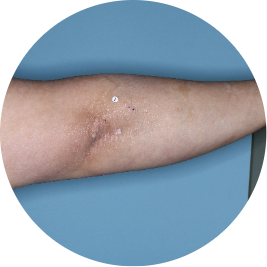
Arm
Actual patient
Identifies as South Asian (Asian)
Before
TREMFYA®
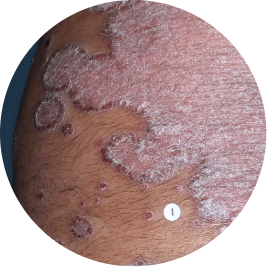
After 3 doses
WEEK 16
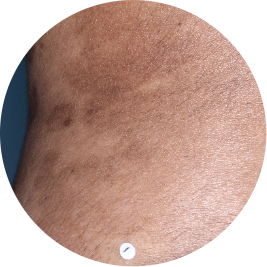
Back
Actual patient
Photos are of real patients with moderate to severe plaque psoriasis who received a single dose of TREMFYA® 100 mg at weeks 0 and 4, and then every 8 weeks. Individual results may vary.
Identifies as South Asian (Asian)
Before
TREMFYA®

After 3 doses
WEEK 16

Back
Actual patient
Photos are of real patients with moderate to severe plaque psoriasis who received a single dose of TREMFYA® 100 mg at weeks 0 and 4, and then every 8 weeks. Individual results may vary.
Identifies as African (Black)
Before
TREMFYA®

After 3 doses
WEEK 16

Back
Actual patient
Identifies as Black
Before
TREMFYA®

After 3 doses
WEEK 16

Scalp
Actual patient
Identifies as Caribbean (Black)
Before
TREMFYA®

After 3 doses
WEEK 16

Chest
Actual patient
Identifies as Caribbean (Black)
Before
TREMFYA®

After 3 doses
WEEK 16

Back
Actual patient
Photos are of real patients with moderate to severe plaque psoriasis who received a single dose of TREMFYA® 100 mg at weeks 0 and 4, and then every 8 weeks. Individual results may vary.
Identifies as Mexican (Hispanic/Latino)
Before
TREMFYA®

After 3 doses
WEEK 16

Leg
Actual patient
Identifies as Mexican (Hispanic/Latino)
Before
TREMFYA®

After 3 doses
WEEK 16

Arm
Actual patient
Identifies as South American (Hispanic/Latino)
Before
TREMFYA®

After 3 doses
WEEK 16

Legs
Actual patient
Identifies as Mexican (Hispanic/Latino)
Before
TREMFYA®

After 3 doses
WEEK 16

Arm
Actual patient
First-of-its-kind
Minority representation has been less than 30% in plaque psoriasis biologic treatment trials. That’s why we launched a first-of-its-kind study specifically across all skin tones.*
*Refers to a dermatology skin tone scale.
Across skin tones
Psoriasis can look different across skin tones, which is something that is often overlooked when considering treatment.
You’re always included
We’re committed to conducting innovative clinical research that includes patients with different skin tones.
Before & After TREMFYA® in Low-BSA Moderate Plaque Psoriasis and High-Impact Areas
See below for before and after photos of real patients with low body surface area (BSA) (2%-15%) moderate plaque psoriasis and plaques in high-impact areas.
Photos are of real patients who received TREMFYA® 100 mg at weeks 0 and 4, and then every 8 weeks.
Individual results may vary.
Scalp
Before
TREMFYA®
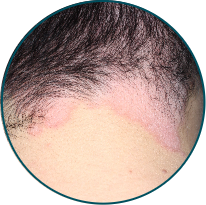
After 3 doses
WEEK 16
Actual patient
Scalp
Before
TREMFYA®
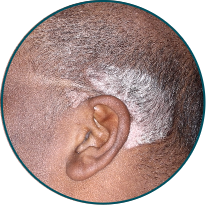
After 3 doses
WEEK 16
Actual patient
Skin folds
Before
TREMFYA®
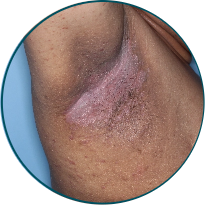
After 3 doses
WEEK 16
Actual patient
Skin folds
Before
TREMFYA®
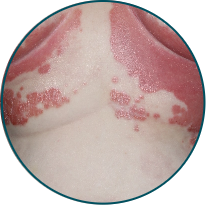
After 3 doses
WEEK 16
Actual patient
High-impact site results at 16 weeks with at least moderate severity
Scalp
At least 7 out of 10 patients on TREMFYA® were rated clear or almost clear of their scalp psoriasis.
Face
At least 8 out of 10 patients on TREMFYA® were rated clear or almost clear of their facial psoriasis.
Genitals
At least 7 out of 10 patients on TREMFYA® were rated clear or almost clear of their genital psoriasis.
Skin folds
At least 8 out of 10 patients on TREMFYA® were rated clear or almost clear of psoriasis in skin fold areas.
TREMFYA® is indicated for the treatment of moderate to severe plaque psoriasis and active psoriatic arthritis.
Psoriatic arthritis is a chronic, or long-term, disease. It affects about 30% of people with psoriasis. While the exact cause is unknown, when you have psoriatic arthritis, your immune system attacks healthy tissues like the skin and joints.
Click the button below to learn more about how TREMFYA® could help treat adults with active psoriatic arthritis.
Get your own English or Spanish brochure and discussion guide
Doctor Discussion Guide
Take this guide with you to your next dermatologist appointment. It’s designed to help patients across all skin tones have an open and honest conversation about their plaque psoriasis.
Guía de Discusión del Médico
Lleve esta guía con usted a su próxima cita con el dermatólogo. Está diseñada para ayudar a los pacientes de todos los tonos de piel a tener una conversación abierta y sincera sobre su psoriasis en placas.
TREMFYA® Safety Information
Ask your doctor about the benefits and risks of TREMFYA®. Prescription medications, including TREMFYA®, have possible risks involved with treatment, so it’s important to discuss them with your doctor.
TREMFYA® may cause serious side effects, including:
- Serious allergic reactions. Stop using TREMFYA® and get emergency medical help right away if you develop any of the following symptoms of a serious allergic reaction:
- fainting, dizziness, feeling lightheaded (low blood pressure)
- swelling of your face, eyelids, lips, mouth, tongue or throat
- trouble breathing or throat tightness
- chest tightness
- skin rash, hives
- itching
- fainting, dizziness, feeling lightheaded
(low blood pressure) - swelling of your face, eyelids, lips,
mouth, tongue or throat
- trouble breathing or throat tightness
- chest tightness
- skin rash, hives
- itching
- Infections. TREMFYA® is a medicine that may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with TREMFYA® and may treat you for TB before you begin treatment with TREMFYA® if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with TREMFYA®.
Tell your healthcare provider right away if you have an infection or have symptoms of an infection, including:
- fever, sweats, or chills
- cough
- shortness of breath
- blood in your phlegm (mucus)
- muscle aches
- warm, red, or painful skin or sores on your body different from your psoriasis
- weight loss
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
- fever, sweats, or chills
- cough
- shortness of breath
- blood in your phlegm (mucus)
- muscle aches
- warm, red, or painful skin or
sores on your body different
from your psoriasis
- weight loss
- diarrhea or stomach pain
- burning when you urinate or
urinating more often than
normal
- Liver problems. With the treatment of Crohn’s disease or ulcerative colitis, your healthcare provider will do blood tests to check your liver before and during treatment with TREMFYA®. Your healthcare provider may stop treatment with TREMFYA® if you develop liver problems. Tell your healthcare provider right away if you notice any of the following symptoms:
- unexplained rash
- vomiting
- tiredness (fatigue)
- yellowing of the skin or the whites of your eyes
- nausea
- stomach pain (abdominal)
- loss of appetite
- dark urine
- unexplained rash
- vomiting
- tiredness (fatigue)
- yellowing of the skin or the whites of your eyes
- nausea
- stomach pain (abdominal)
- loss of appetite
- dark urine
These are not all the possible side effects of TREMFYA®.
Please read the Important Safety Information located at the bottom of the screen and the Medication Guide for TREMFYA® to learn more about these and other risks for TREMFYA®. Discuss any questions you have with your doctor.
TREMFYA® may cause serious side effects including:
- See “What is the most important information I should know about TREMFYA®?”The most common side effects of TREMFYA® include:
- respiratory tract infections
- joint pain (arthralgia)
- fungal skin infections
- bronchitis
- headache
- diarrhea
- herpes simplex infections
- injection site reactions
- stomach flu (gastroenteritis)
- stomach pain
- respiratory tract infections
- joint pain (arthralgia)
- fungal skin infections
- bronchitis
- headache
- diarrhea
- herpes simplex infections
- injection site reactions
- stomach flu (gastroenteritis)
- stomach pain
These are not all the possible side effects of TREMFYA®. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.