For adults with moderately to severely active ulcerative colitis (UC).
TREMFYA®
DOSING
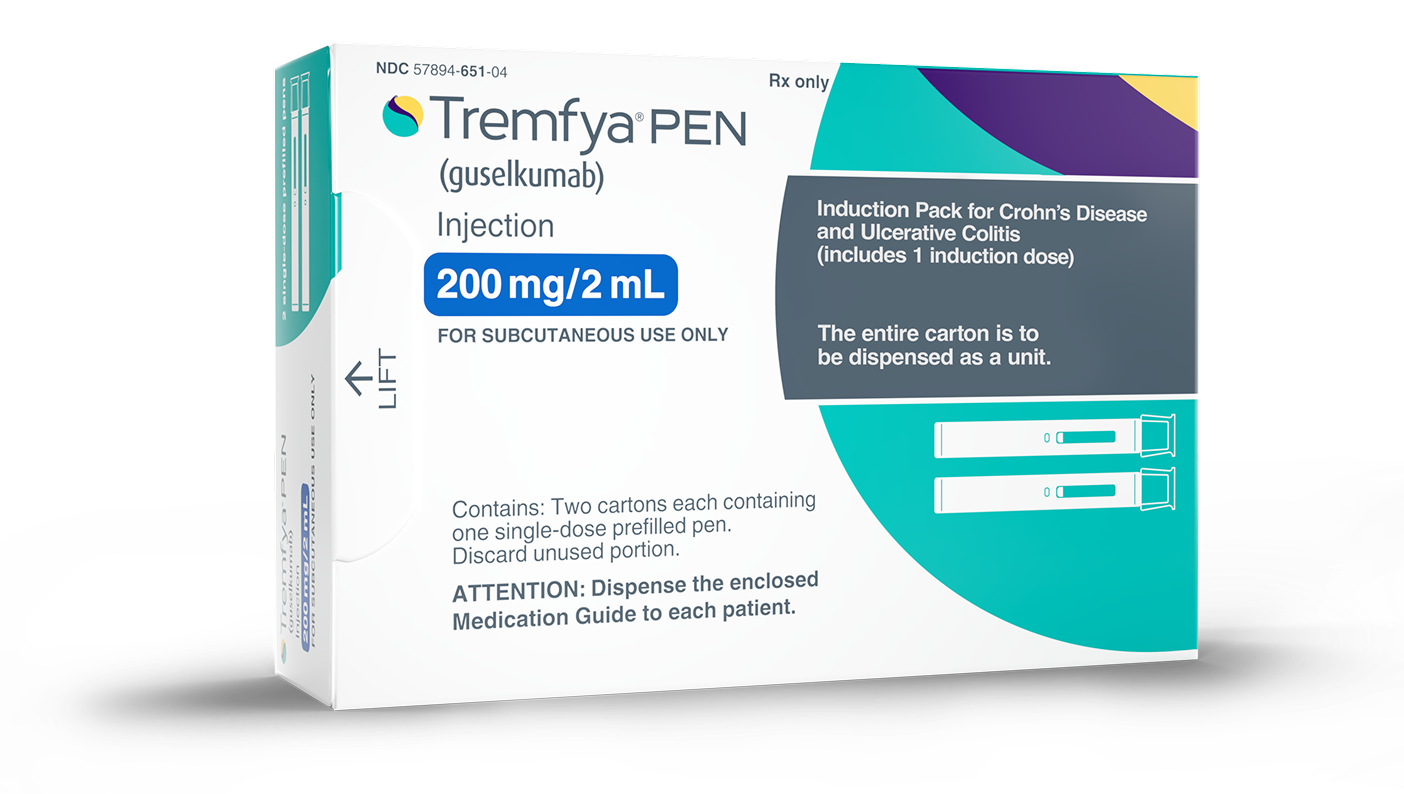
TREMFYA® is the only IL-23 blocker for UC to offer self-administration from the start
If your healthcare provider decides that you or a caregiver may be able to give your injections of TREMFYA® at home, you should receive training on the right way to prepare and inject TREMFYA®. Do not try to inject yourself until you have been trained by your healthcare provider.
There are two options to get started with TREMFYA®:
Injections under the skin with subcutaneous (SC) injections
OR
Through a vein in the arm with intravenous (IV) infusions
INITIAL DOSES
SC INJECTIONS under the skin
Your first 3 doses may be taken as 2 SC injections every 4 weeks.*
WEEK 0
400 MG
WEEK 4
400 MG
WEEK 8
400 MG
*Each 400 mg dosage will be given as two consecutive injections of 200 mg.
OR
INTRAVENOUS (IV) INFUSION through a vein in the arm
You may be given an IV infusion every 4 weeks at a healthcare facility. It takes at least 1 hour for each dose.
WEEK 0
200 MG
WEEK 4
200 MG
WEEK 8
200 MG
MAINTENANCE
DOSES
DOSES
SC INJECTIONS
After finishing your initial doses, you will receive TREMFYA® subcutaneously (under the skin) as:
- A 200 mg dose every 4 weeks starting at week 12
OR
- A 100 mg dose every 8 weeks starting at week 16
WEEK 12
AND EVERY 4 WEEKS AFTER
200 MG
OR
WEEK 16
AND EVERY 8 WEEKS AFTER
100 MG
Your healthcare provider will prescribe the right dose for you. Use TREMFYA® exactly as prescribed.
IMPORTANT: Please read the Instructions for Use before using TREMFYA®, and each time you get a refill. There may be new information. These instructions do not take the place of talking with your healthcare provider about your medical condition or your treatment.
IL=interleukin.

Administration options for TREMFYA®
Once you have had your first three initial doses, you will take TREMFYA® as a subcutaneous injection.
After your healthcare provider decides how much medicine you’ll be injecting, and how often, you can discuss which injection device may be appropriate for you.

TREMFYA® PEN
200 mg/2 mL or 100 mg/mL
Not actual size 200 mg/2 mL TREMFYA® PEN pictured


Prefilled Syringe
200 mg/2 mL or 100 mg/mL
Not actual size 200 mg/2 mL prefilled syringe pictured
The One-Press patient-controlled injector is available as an alternative device option.
Talk to your healthcare provider about which administration option is right for you.


~910
~9 out of 10 patients with UC or CD would choose a subcutaneous injection pen over an SC on-body injection device.*†‡
~810
~8 out of 10 patients with UC or CD would choose a subcutaneous injection pen over an IV infusion.*†
*Based on a validated third-party 2025 market research survey† of 506 patients, including both bio-naïve (patients who were new to a biologic) and bio-experienced (patients who previously received a biologic). This survey reflects participant opinions. It did not evaluate efficacy or safety. While these factors are important, there are additional considerations for selecting treatment. Talk to your healthcare provider about treatment options and what might be right for you.
†Survey question: Please read each description carefully and select which one you would prefer to use for your Crohn’s disease/ulcerative colitis.
‡TREMFYA® does not offer an SC on-body injection device.
CD=Crohn’s disease.
If you’re starting TREMFYA® subcutaneously, you may receive a TREMFYA® Induction Pack for your initial doses.
Explore resources designed to help you understand where and how to inject TREMFYA® with the prefilled pen.
Watch the TREMFYA® PEN demonstration video